Hydrogenation without hydrogen: Efficient catalysis in a stable emulsion gel
UJ researchers take a novel step to change hydrogenation into a safe, low energy process. They use a very stable three phase emulsion to transform a toxic waste product into valuable feedstock. The process does not need flammable, compressed hydrogen gas.
The emulsion catalysis hydrogenates nitrobenzene efficiently at room temperature to output aniline. Aniline is widely used in the pharmaceutical industry. The bi-metallic hydrogenation catalyst is fully recovered afterwards.
Without hydrogenation, it would not be possible to manufacture many of today's medicines. It is a backbone process for the pharmaceutical and chemical industries. But hydrogen is expensive. The safety measures to prevent explosions in factories and laboratories are costly also.
However, if compressed hydrogen is not needed at all, significant savings are possible. It also means that many chemical processes can be much safer and easier to work with.
Chemists from the University of Johannesburg have demonstrated this, in research published in Colloids and Surfaces.
They converted nitrobenzene into aniline, using a catalyzed hydrogenation process in a Pickering emulsion.
The emulsion process has the potential to be a much safer industrial hydrogenation process than those currently in use.
"Pickering emulsions have been around for 150 years. But using them for catalysis only emerged in 2014," says Prof Reinout Meijboom. Meijboom is a researcher at the department of Chemical Sciences.
Yogurt, is an example of a Pickering emulsion. Such an emulsion is a mix of particles that readily dissolve in water, and particles that readily dissolve in oil. What makes yogurt a Pickering emulsion is that it also contains enzymes, which are solid particles that do not dissolve.
Nitrobenzene is produced in huge amounts globally as waste from chemical manufacturing. It is a highly toxic, persistent organic pollutant described by the WHO, EPA and CDC, among others.
The manufacture of polyurethanes uses nitrobenzene as an intermediate. It is also used as a solvent in petroleum refining. The wastewater of dye manufacturers often contains nitrobenzene. It is an oily liquid and presents a fire hazard.
Aniline is an industrially significant commodity. It is a feedstock for a vast number of chemical products, including many medicines.
The process the researchers designed uses toluene to dissolve nitrobenzene. This forms the first, organic or toluene phase of the process. For the second aqueous phase, they dissolved sodium borohydride in water.
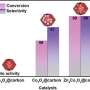
The catalyst is the third phase in the process. It consists of modified silica microspheres and Palladium. They also used a bimetallic catalyst, where Palladium is combined with Cobalt or Nickel.
If the three phases are added together, but not mixed into an emulsion, the combination can be stored for days or weeks, says Meijboom. A small amount of hydrogenation takes place, but the process only really gets going once a proper emulsion is formed.
The catalyst also acts as a stabilizing emulsifier.
When the three phases are mixed into an emulsion, the catalyst kick-starts the hydrogenation process. The formation of the emulsion takes a few seconds. The reaction takes about two hours at laboratory scale.
The hydrogen needed for hydrogenation is supplied by the dissolved sodium borohydride. Hydrogenation happens efficiently at room temperature, which saves energy.
There is no need for stored or piped hydrogen. This removes most of the explosion risk from the process.
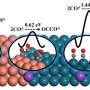
The three-phase process in a Pickering emulsion has the advantage of a much bigger catalytic surface, compared to a single phase or two phase process, says Meijboom.
The catalytic efficiency can be tuned by adjusting the volume ratio of the toluene and water phases in the Pickering emulsion system.
"Each drop of toluene and nitrobenzene solution in the emulsion effectively becomes a microreactor. This is how the process can be tuned to be efficient at room temperature," he adds.
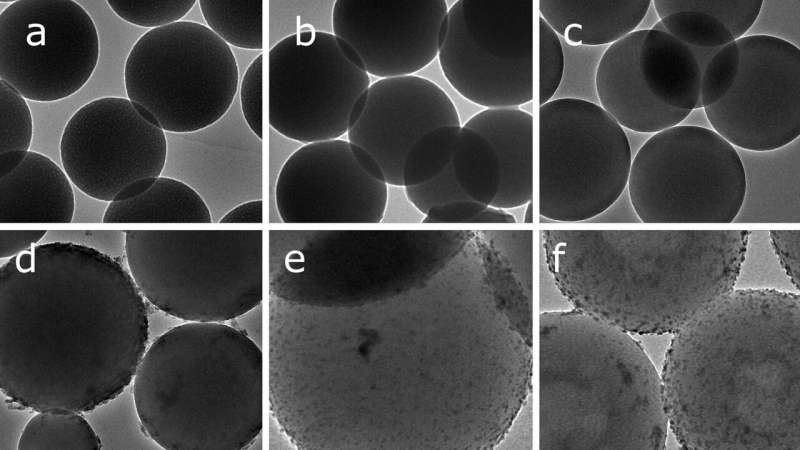
After the hydrogenation completes, the resulting emulsion is stable enough for storage for a few days, before separating out the aniline.
The study is the first efficient use of a bimetallic palladium catalyst for the hydrogenation of an aromatic compound in a water-based Pickering emulsion system, says Mr. Peter Dele Fapojuwo. He is a Postgraduate researcher at the department.
"By adding nickel or cobalt to the catalyst, we improved the dispersion of the Palladium on the surface of the emulsifier," he adds.
Palladium is much more expensive than nickel or cobalt, so the use of the bimetallic catalyst further reduces costs.
"The use of solid particles as both catalysts and emulsifier, or stabilizer, poses less threat to the environment compared to conventional surfactant. Their composition is less toxic," says Fapojuwo.
The reaction platform is significantly safer when using sodium borohydride as the reductant, rather than hydrogen, he adds.
"Hydrogenation using petroleum-derived hydrogen is neither totally environmentally-friendly nor economically viable. It requires high-pressure hydrogen, which demands expensive reactor equipment. That increases process costs," he adds.
"In theory, this process can be adapted to keeping one phase in a fixed-bed reactor and doing flow synthesis. The result would be a continuous process for catalytic reactions between two immiscible phases," says Meijboom.
"This is proof of principle phase. We're working towards generalizing the process," he says.
"We have designed a process that can be expanded to a range of industrially important reactions.
"By using emulsion chemistry, we have one system where catalyst, emulsifier, aqueous and organic phase all mix up into an extremely stable system," says Meijboom.
More information: Dele Peter Fapojuwo et al, Bimetallic PdM (M = Co, Ni) catalyzed hydrogenation of nitrobenzene at the water/oil interface in a Pickering emulsion, Colloids and Surfaces A: Physicochemical and Engineering Aspects (2021). DOI: 10.1016/j.colsurfa.2021.126513
Provided by University of Johannesburg